-
Pro's OnlyElectricians Arms Electrician Talk How to Access The Arms Domestic Electrician Industrial Electricians Wiring, Theories, Regulations Engineering Chat Periodic Testing Problems Electricians Downloads Commercial Electricians Security (Access-Only) Access Private Area Business Related Advice Certification Schemes Electrical & PAT Testing
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
- Thread starter Mikegh
- Start date
Most metals with aluminium cause corrosion if any damp present, since Al is so high on the reactivity list (at least, of metals you typically encounter in electrical work). How bad it is depends on the difference in electrode potential:
 en.wikipedia.org
Zinc plating / galvanising is designed so the coating sacrifices itself to protect the base metal, such as steel, for corroding itself. Zinc is OK with aluminium, but most other metals such as stainless steel, brass and copper really go for it. Same applies if you have, say, a brass fixing on galvanised metal - it will accelerate the loss of the plating.
en.wikipedia.org
Zinc plating / galvanising is designed so the coating sacrifices itself to protect the base metal, such as steel, for corroding itself. Zinc is OK with aluminium, but most other metals such as stainless steel, brass and copper really go for it. Same applies if you have, say, a brass fixing on galvanised metal - it will accelerate the loss of the plating.
Here is a chart showing what is a good mix (white) and what to avoid (red):
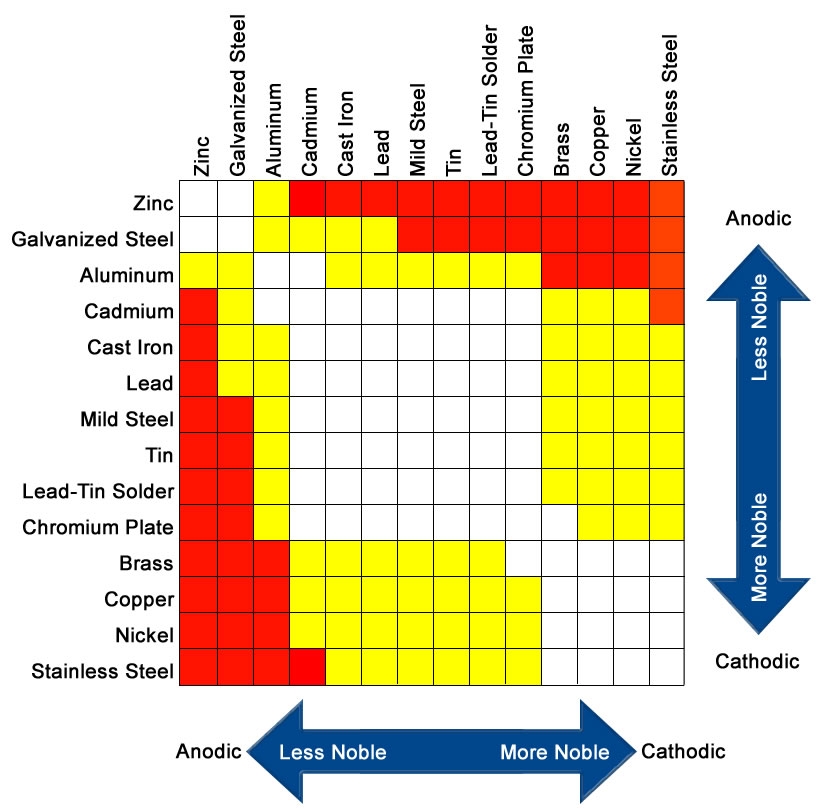
In practice you can't always avoid bad combinations so the next-best option is to exclude water+oxygen from the joint so it is not corroded.
You can use lubricating grease on mechanical fasteners, in fact you generally always should, especially on stainless that is prone to galling. But for electrical work you don't want any extreme-pressure lubricants that stops metal-metal contact for obvious reasons! Here you can get special compounds for aluminium use such as Noalox / Penetrox which have (I think) metallic zinc particles in them, but Vaseline is a good option for general use, so long as not high temperature. Traditionally that was used on car battery terminals to avoid corrosion issues.
Galvanic series - Wikipedia
Here is a chart showing what is a good mix (white) and what to avoid (red):
In practice you can't always avoid bad combinations so the next-best option is to exclude water+oxygen from the joint so it is not corroded.
You can use lubricating grease on mechanical fasteners, in fact you generally always should, especially on stainless that is prone to galling. But for electrical work you don't want any extreme-pressure lubricants that stops metal-metal contact for obvious reasons! Here you can get special compounds for aluminium use such as Noalox / Penetrox which have (I think) metallic zinc particles in them, but Vaseline is a good option for general use, so long as not high temperature. Traditionally that was used on car battery terminals to avoid corrosion issues.
N
nicebutdim
Zinc plating / galvanising is designed so the coating sacrifices itself to protect the base metal, such as steel, for corroding itself. Zinc is OK with aluminium, but most other metals such as stainless steel, brass and copper really go for it. Same applies if you have, say, a brass fixing on galvanised metal - it will accelerate the loss of the plating.
Odd that we regularly use brass bushes with galv conduit fittings.
Fine what it is dry!Odd that we regularly use brass bushes with galv conduit fittings.
I guess for outdoor use, etc, the plated steel ones would be better. But #1 rule is keep it lubricated if you ever want to change it in the future.
The threaded part is generally not galvanised.Odd that we regularly use brass bushes with galv conduit fittings.
N
nicebutdim
But #1 rule is keep it lubricated if you ever want to change it in the future.
Good advice in general.
N
nicebutdim
The threaded part is generally not galvanised.
Would cheap couplers be threaded after galvanising? Never paid much attention to what goes on inside, but will have to take a look now.
Never seen a coupler galvanised internally.
You could put it all around an assembled joint, but if you apply first and then assemble you get it filling all of the tiny cracks and surface imperfections to keep the moisture out. The worst corrosion tends to be closest to the metal-metal joint where they differ, so by making sure that is covered at a microscopic level you get better protection.What's the idea behind putting Vaseline between them ?
Would it not be around them ?
Covering with any sort of grease, or even a spray with WD40, etc, afterwards will help keep moisture off. It is the flow of ions within any moisture that is responsible for the effect, hence why it is far worse if the moisture has salt (near sea) or chlorine (near swimming pool), etc, dissolved in it.
Swimming pool plant rooms are a nightmare!To be fair, chlorine will eat away at most things without any other help!
Similar threads
- Replies
- 19
- Views
- 1K
OFFICIAL SPONSORS









These Official Forum Sponsors May Provide Discounts to Regular Forum Members - If you would like to sponsor us then CLICK HERE and post a thread with who you are, and we'll send you some stats etc
Advert
Thread Information
- Title
- Galvanic Corrosion Electrical Work
- Prefix
- N/A
- Forum
- UK Electrical Forum
- Replies
- 15
- Unsolved
- --
Thread Tags
Advert
Advert
TrueNAS JBOD Storage Server
-
-
Understanding TrueNAS JBOD Servers: A Comprehensive Guide
- Started by Dan
- Replies: 8
-
